Blockchain | 01 Sep 2023 | 16 min
Disrupting Pharma Industry with Blockchain

Learn how Blockchain can enhance transparency in the pharma industry
As the pharma industry evolves and more generic drugs emerge, ensuring both generic and brands are protected becomes essential. What’s more, consumers today increasingly demand more information on medications. And rightfully so, with critical medications, the stakes are always high. When it comes to customer satisfaction, trust and transparency can go a long way. For the pharma world, this is a tedious affair.
The confluence of cutting-edge technologies and regulatory standards has paved the way for innovative solutions.
In this blog, we delve into the intricate world of blockchain in the pharma world. We explore how blockchain technology and Track and Trace Applications can transform processes, determine access to stakeholders, and revolutionize transparency and compliance within the pharmaceutical industry.
Why is blockchain so important to the pharmaceutical industry?
A three-word answer would be ‘supply chain vulnerability’. In an otherwise competitive world, blockchain allows companies with competing interests to work together, reduce friction, detect frauds, improve product authenticity, and get all those involved to trust data.
Drug approvals can be tricky since generic drug manufacturers require the Food and Drug Association (FDA) to anoint them the rights to manufacture brand drugs. Here are three factors that HCPs, physician, pharmacists, patients, and routine consumers look for while purchasing or placing an order for generic drugs.
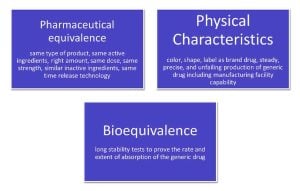
Fig 01: Factors to look for while purchasing generic drugs
Here is where Blockchain track and trace apps (T&T) can make workflows more effective and effectively maintain transparency:
Thus, the three key factors—pharmaceutical equivalence, bioequivalence, and physical characteristics—are critical considerations for healthcare professionals, physicians, pharmacists, patients, and everyday consumers when selecting or ordering generic alternatives to brand-name medications.
By warranting that these three aspects are in place, generic drug companies can improve the chances of patient acceptance drastically.
Additionally, integrating blockchain and Track and Trace Applications elevates transparency and trust by securely recording every step, from manufacturing to bioequivalence tests, on an immutable ledger. This synergy ensures reliable data access for stakeholders. This technological collaboration guarantees medication consistency, minimizes errors, and transforms the healthcare landscape, harmonizing advanced technologies with crucial pharmaceutical factors
As a generic drug manufacturer capturing the attention of the drug market and consumers and HCP will be a race and one who shows transparency, robust data, and sound clinical results are key factors to success, even alongside competitive pricing.
Instead of going the patents and exclusivity route, what can be theirs to take is the post-patent expiry stage.
During this phase, multiple generic drug manufacturers receive approval to create alternatives to brand drugs. This involves various steps including:
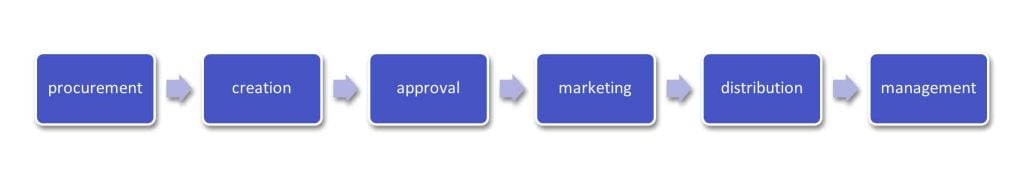
Fig 02: Steps to receive approval to create alternative drugs
These processes can be monitored, highlighted, enhanced, and streamlined with transparency, privacy, and real-time updates through Finboot’s Track and Trace application.
A strong network of suppliers and retail pharmacies offers several benefits to pharmaceutical companies. The pandemic stands testament of this requirement, where we witnessed severe supply chain disruption, drug wastage, and inflated prices due to middlemen.
A network enhances product visibility, establishes a strong customer base, and enables real-time data collection to make informed decisions. The unification and analysis of such powerful data pharma companies can grow at a rapid pace and respond in the most appropriate and swift manner to market dynamics.

Fig 03: Blockchain for pharmaceutical supply chain
Mapping, tracking, and tracing loopholes and critical issues like counterfeits entering the market through example singular retail pharmacists can be kept in check with the help of a strong network.
In the pharmaceutical industry where data collection becomes the most critical decider of competitive advantage, blockchain can play a vital role.
When both suppliers and pharmacists are added to the blockchain network the accountability increases. Finboot’s Blockchain-based MARCO platform helps keep track of the number of medicines supplied to and sold by pharmacists. This means seamless tracking of supply and sale of drugs becomes possible and the distinction between counterfeit and low-quality drugs also comes to light.
By keeping a record of this on T&T apps, suppliers and pharmacists can update their data and attach it to the workflows, which in turn can be viewed by pharma companies.
The data collected can be analyzed by manufacturers to optimize production rates, estimate future purchase orders, and make key decisions based on defects, market trends, and demand forecasts.
Research points out that most HCPs feel the need to increase their online interaction with pharma companies directly.
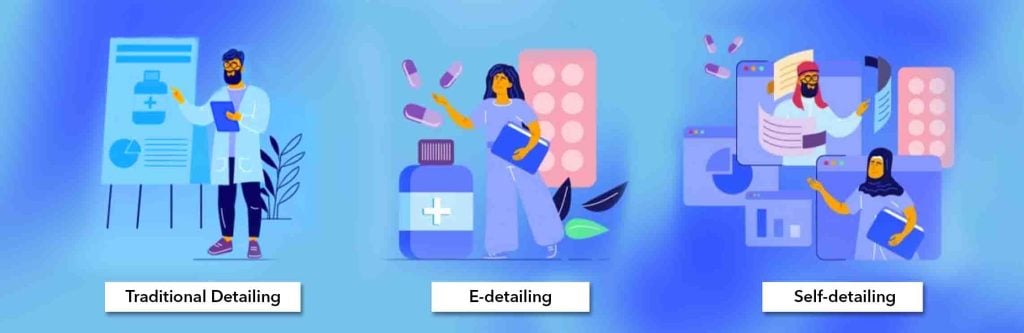
Fig 04: Types of detailing in pharmaceuticals
This signals that HCPs themselves can participate in e-detailing through blogs, virtual interactive sessions and the like with the pharma company or its subset self-detailing through self-paced learning of materials provided during e-detailing as opposed to traditional detailing through sales or medical representatives.
Blockchain technology offers solutions to streamline pharmaceutical marketing, focusing on reducing the need for salespersons and enhancing personalization. This is one technology, that I think, champions a trustless ecosystem, and can replace middlemen.
Immutable drug details on the blockchain enable HCPs to access accurate information independently, leading to more engaged interactions. This process increases the likelihood of HCPs participating in interactive sessions, optimizing resource allocation for pharma companies.
Working with HCPs can prove to be challenging for pharma companies due to lack of targeting opportunities. Creating an effective marketing strategy and forging a connection with them and then presenting data seems to be a long-drawn game.
But, by utilizing blockchain technology, tamper-proof data can be made available to end users, consumers, HCPs, and patients. Attaching details of clinical trials, statistics, medical trial information in the workflows of T&T apps can help build that much needed trust.
Finboot’s MARCO platform and T&T app through blockchain help display immutable data viz. FDA approvals, trial details, side effects & chemical composition details, and testimonials via a single QR code.
Thus, the Track and Trace application within the MARCO platform ensures transparent and secure data display through QR codes, improving information dissemination and stakeholder communication.
Tracking and tracing drugs throughout the pharmaceutical supply chain is essential for ensuring drug quality, safety, and availability.
The complex process involves various stakeholders from manufacturers to patients.
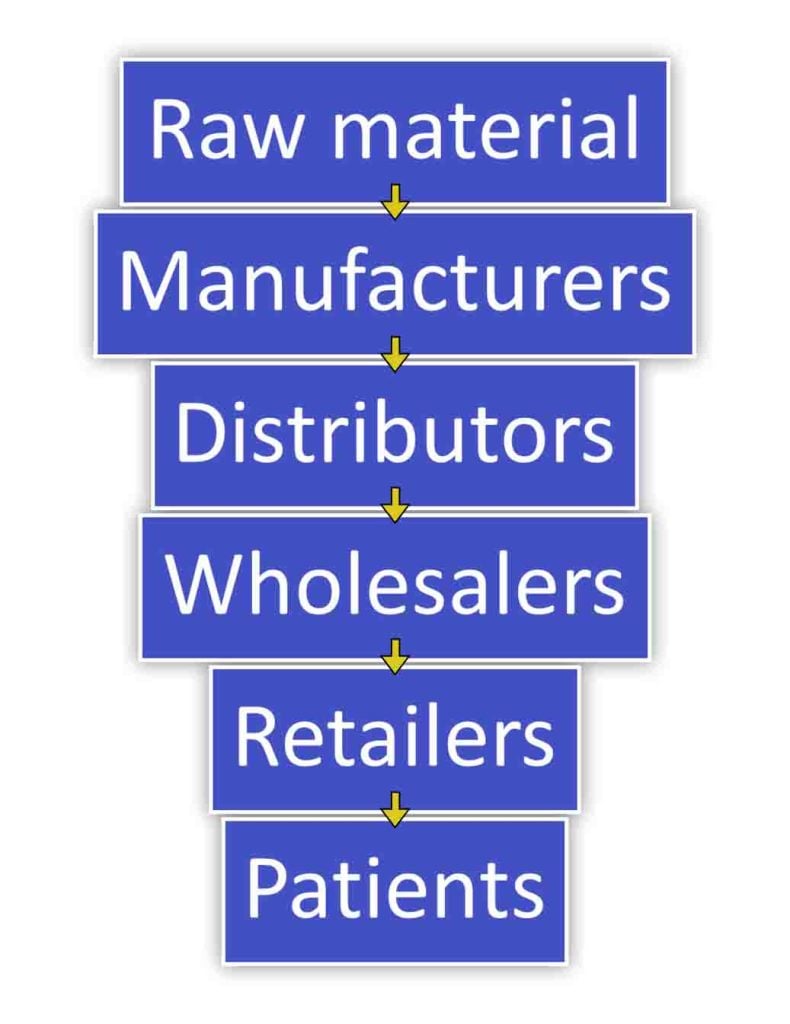
Fig 05: Lifecyle of a drug
The pharmaceutical sector relies on strict regulations and collaboration to reduce counterfeit risks, ensure timely delivery, and minimize waste. Transportation and logistics are critical for effective drug delivery.
By maintaining detailed records and utilizing technologies like blockchain and tracking applications, companies can ensure temperature control, efficient dispatch planning, inventory management, and regulatory compliance.
Transparently showcasing manufacturing practices and standards on blockchain establishes trust among consumers, healthcare professionals, and international authorities. Compliance with Current Good Manufacturing Practices (CGMP) is crucial to prevent adulteration.
Blockchain through T&T apps can empower pharma companies:

Reach out to us at Nitor Infotech to learn more about our blockchain capabilities.

we'll keep you in the loop with everything that's trending in the tech world.
